Revealing the Potential of the Immune System in Cancer
The immune system is capable of recognizing and eliminating tumor cells
Innate and adaptive immunity act as a complementary network of self-defense against foreign threats, including pathogens and cancer.1 An essential feature of the immune system is its ability to recognize foreign threats (nonself) as distinct from normal cells (self).2-4 Despite originating from normal cells, tumor cells can be recognized as nonself because of their capacity to elicit the production of tumor antigens. Neoantigens, a class of tumor antigens, are derived from self proteins but are altered or modified, making them unique to the tumor and previously undetected by the immune system.5,6 Both the innate and adaptive immune systems have the ability to recognize self from nonself.
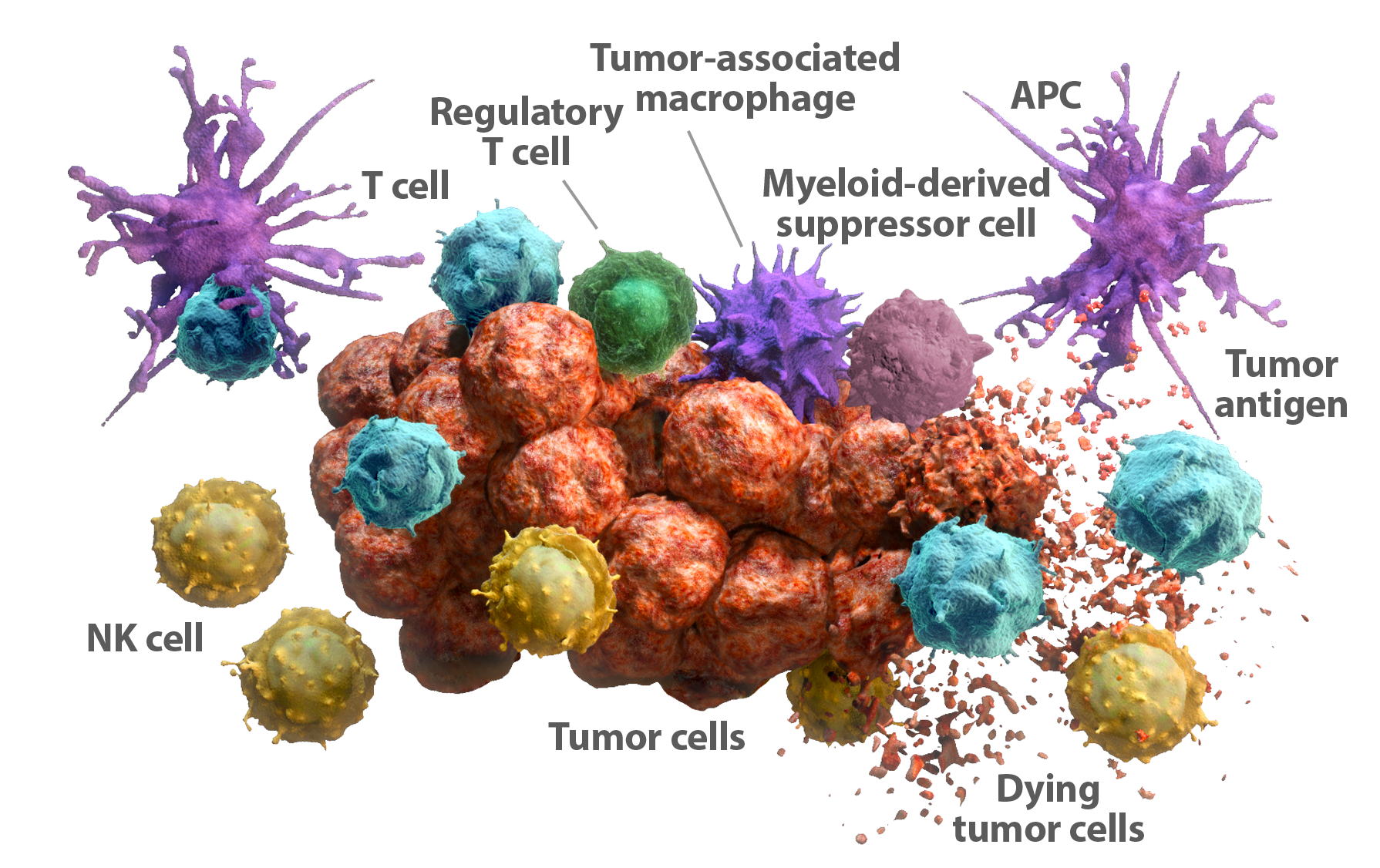
The innate immune response is the body’s first line of defense against pathogens and cancer.1,7,8 It is constantly on alert and can rapidly identify and attack tumor cells. The speed and scope of its response is not limited by antigen specificity, but it is regulated to protect normal cells. The innate immune response recognizes activating and inhibitory signals from target cells to distinguish self from nonself. This distinction allows for the elimination of tumor cells, while sparing normal cells.9-11 Natural Killer (NK) cells are the main effector cells of the innate immune system.
The adaptive immune response is antigen specific and able to produce a durable response.1,8 It recognizes tumor antigens through interaction with antigen-presenting cells (APCs) such as dendritic cells. Although not immediate, once the adaptive immune response is activated, it can be sustained through an immune memory response.12 Cytotoxic T cells are effector cells of the adaptive immune system.
When tumor cell death is caused by the innate immune response, tumor antigens are released, which can be recognized by the adaptive immune response.13 Tumor neoantigens can be derived from mutations at the DNA level. The collective number of mutations within the tumor is known as the mutational load.6 Some tumors have a higher mutational load than others.14,15 Infiltration of cytotoxic T cells into the tumor may be closely linked to a high mutational load—the more tumor neoantigens available, the greater the potential for T-cell activation and infiltrations.16,17
The key stages of the antitumor immune response
In both the innate and adaptive immune responses, as described above, immune cells have the potential to recognize and eliminate abnormal cells such as tumor cells.
There are 3 principal stages in this process:
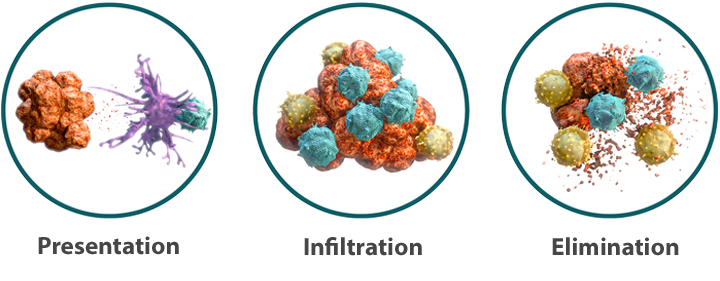

- Presentation: The innate immune system rapidly identifies and attacks tumor cells. The resulting tumor cell death releases tumor antigens, which can activate the cytotoxic T cells of the adaptive immune system13,18
- Infiltration: Tumor antigens and other factors attract immune cells to the tumor site, where they invade and attack18
- Elimination: Activated cytotoxic T cells recognize tumor cells as the source of the antigen and target them for elimination18
The antitumor activity of NK cells and cytotoxic T cells is regulated through a network of activating and inhibitory signaling pathways.4,19,20 Activating pathways trigger an immune response. Inhibitory pathways, such as immune checkpoint pathways, provide a natural counterbalance to immune activation. This balance between activating and inhibitory pathways normally enables the immune system to attack tumor cells, while sparing healthy cells.20
References
1. Warrington R, Watson W, Kim HL, et al. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2011;7(suppl 1):S1. 2. Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280(5361):243-248. 3. Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22(6):1136-1151. 4. Leung J, Suh WK. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw. 2014;14(6):265-276. 5. Lu Y-C, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28(1):22-27. 6. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69-74. 7. Cheng M, Chen Y, Xiao W, et al. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230-252. 8. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11-22. 9. Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114(13):2657-2666. 10. Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132(2):315-325. 11. Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol. 2015;15:243-254. 12. Lau LL, Jamieson BD, Somasundaram T, et al. Cytotoxic T-cell memory without antigen. Nature. 1994;369(6482):648-652. 13. Liu C, Lou Y, Lizée G, et al. Plasmacytoid dendritic cells induce NK cell–dependent, tumor antigen–specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118(3):1165-1175. 14. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415-421. 15. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. 16. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27(8):1492-1504. 17. Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15(4):857-865. 18. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10. 19. Long EO, Kim HS, Liu D, et al. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227-258. 20. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.