Adaptive Immunity: Specific Defenses
The adaptive immune response is durable and antigen-dependent
Adaptive immunity is antigen-dependent and antigen-specific and able to produce a durable response.1 In cancer, tumor antigens such as those released by tumor cell death are derived from mutated or modified self proteins.2 Tumors that have more somatic mutations—known as a higher mutational load-may have the potential to generate a larger number of neoantigens.3 When more tumor antigens are present in the tumor microenvironment, there may be a greater opportunity to stimulate T-cell activity.3,4
Cytotoxic T cells, the primary effector cells of the adaptive immune response, are activated by nonself antigens, including tumor antigens, in secondary lymphoid organs such as lymph nodes.1,5 Once activated, cytotoxic T cells proliferate, migrate to the location of the antigen, and infiltrate it, directly initiating cell death.6 Unlike the innate immune response, adaptive immunity is not immediate but can be sustained through a memory cell response, which includes memory T cells.1,7
T cells are activated by tumor antigens
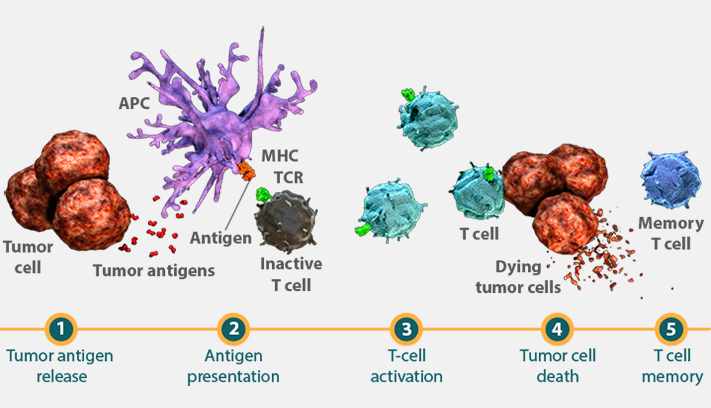
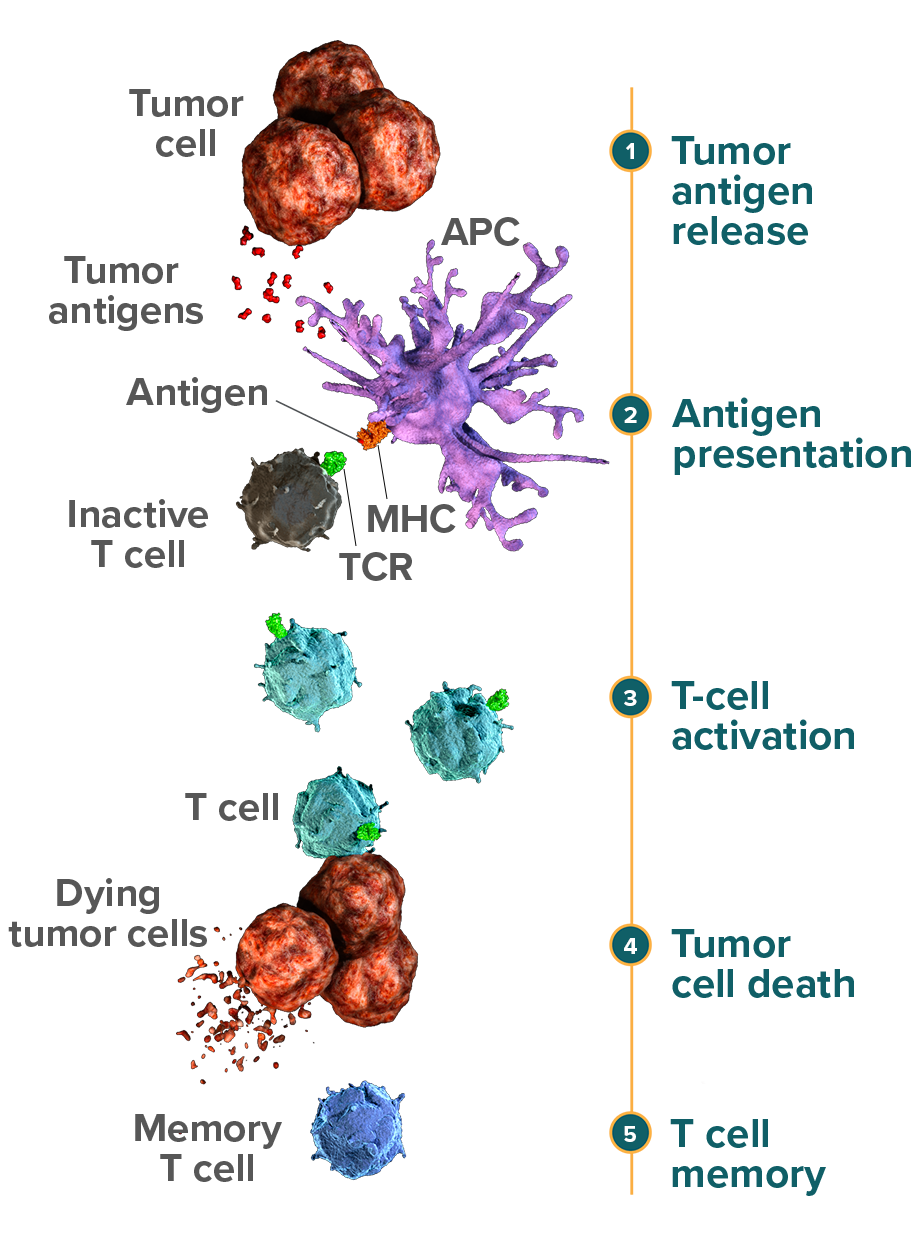
Antigen-presenting cells (APCs) express cell surface proteins called major histocompatibility complexes (MHCs) that present fragments of processed antigen to the T cell.1,8 T-cell receptors (TCRs) on the surface of inactive T cells recognize the presented MHC-antigen complex, which initiates activation and proliferation of cytotoxic T cells.1,9 Antigen recognition later causes activated T cells to migrate to, and infiltrate, the tumor site. Following infiltration into the tumor, cytotoxic T cells release secreted factors capable of promoting tumor cell death.1 Preclinical data suggest that a higher mutational load may be associated with an increased likelihood of greater infiltration of cytotoxic T cells.4
Upon resolution of the immune attack, cytotoxic T cells either die or differentiate into memory T cells that persist long-term.10 Memory T cells are able to re-recognize the antigen, providing the potential for a subsequent immune response.1,7
Activated and memory T cells can migrate throughout the body in search of antigens
To identify and eliminate tumor cells, cytotoxic and memory T cells must be able to scan peripheral tissues in search of their unique activating antigen.6,9 To make this possible, activated T cells upregulate factors that enable them to recognize threats and migrate through blood vessel walls and into affected tissues.11,12
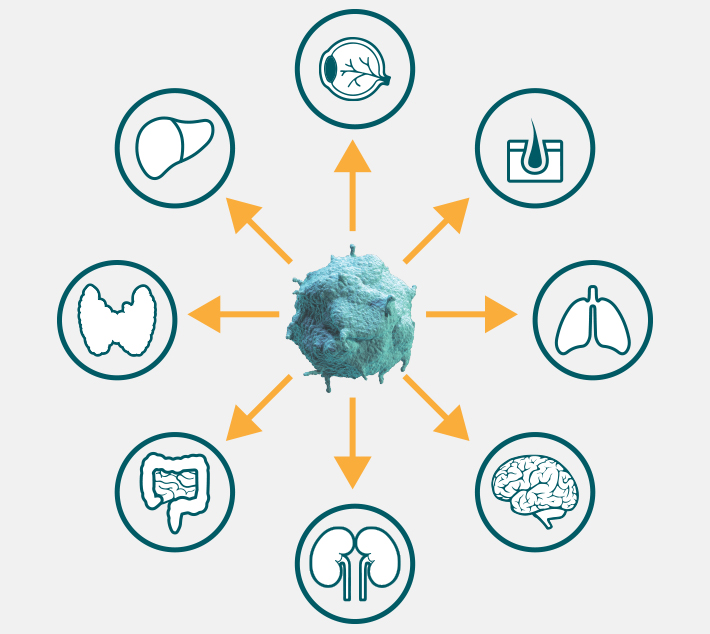

T-cell migration occurs across non-lymphoid tissues, with documented trafficking to even particularly selective tissues such as the eye and brain.13-19 Though the brain was once thought to be “immune privileged,” data suggest that the blood-brain barrier can be "leaky," allowing for the movement of T cells and other immune molecules. This mobility enables activated cytotoxic T cells to patrol for antigens and infiltrate tumors in the brain.13,20 After the activated cytotoxic T cell population diminishes, memory T cells remain capable of trafficking to surrounding tissues in the event of antigen reoccurence.14
References
1. Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2011;7(suppl 1):S1. 2. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69-74. 3. Liontos M, Anastasiou I, Bamias A, Dimopoulos M-A. DNA damage, tumor mutational load and their impact on immune responses against cancer. Ann Transl Med. 2016;4(14):264. doi:10.21037/atm.2016.07.11. 4. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27(8):1492-1504. 5. Mondino A, Khoruts A, Jenkins MK. The anatomy of T-cell activation and tolerance. Proc Natl Acad Sci USA. 1996;93(6):2245-2252. 6. Krummel MF. T cell migration, search strategies and mechanisms. Nat Rev Immunol. 2016;16(3):193-201. 7. Lau LL, Jamieson BD, Somasundaram T, et al. Cytotoxic T-cell memory without antigen. Nature. 1994;369(6482):648-652. 8. Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86-89. 9. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev Cancer. 2012;12(4):252-264. 10. Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13(5):309-320. 11. Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168-7174. 12. Ferguson AR, Engelhard VH. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. J Immunol. 2010;184(8):4079-4086. 13. Masopust D, Vezys V, Usherwood EJ, et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172(8):4875-4882. 14. Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9(3):153-161. 15. Hirata T, Furie BC, Furie B. P-, E-, and L-selectin mediate migration of activated CD8 T lymphocytes into inflamed skin. J Immunol. 2002;169(8):4307-4313. 16. Wekerle H, Sun D. Fragile privileges: autoimmunity in brain and eye. Acta Pharmacol Sin. 2010;31(9):1141-1148. 17. Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol. 2006;6(9):682-692. 18. Walch JM, Zeng Q, Li Q, et al. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest. 2013;123(6):2663-2671. 19. Dace DS, Chen PW, Niederkorn JY. CD8+ T cells circumvent immune privilege in the eye and mediate intraocular tumor rejection by a TNF-α-dependent mechanism. J Immunol. 2007;178(10):6115-6122. 20. Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich P-Y, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179(2):845-853.