Realizing the Potential of Immuno-Oncology Research
Depth of evidence for the immune response to cancer
Both solid tumors and hematologic malignancies are able to induce an immune response that can regulate their initial growth. This ability is known as tumor immunogenicity.1,2
Robust evidence supports the body’s ability to recognize and attack cancer through immune cell presentation, infiltration, and elimination.
Presentation
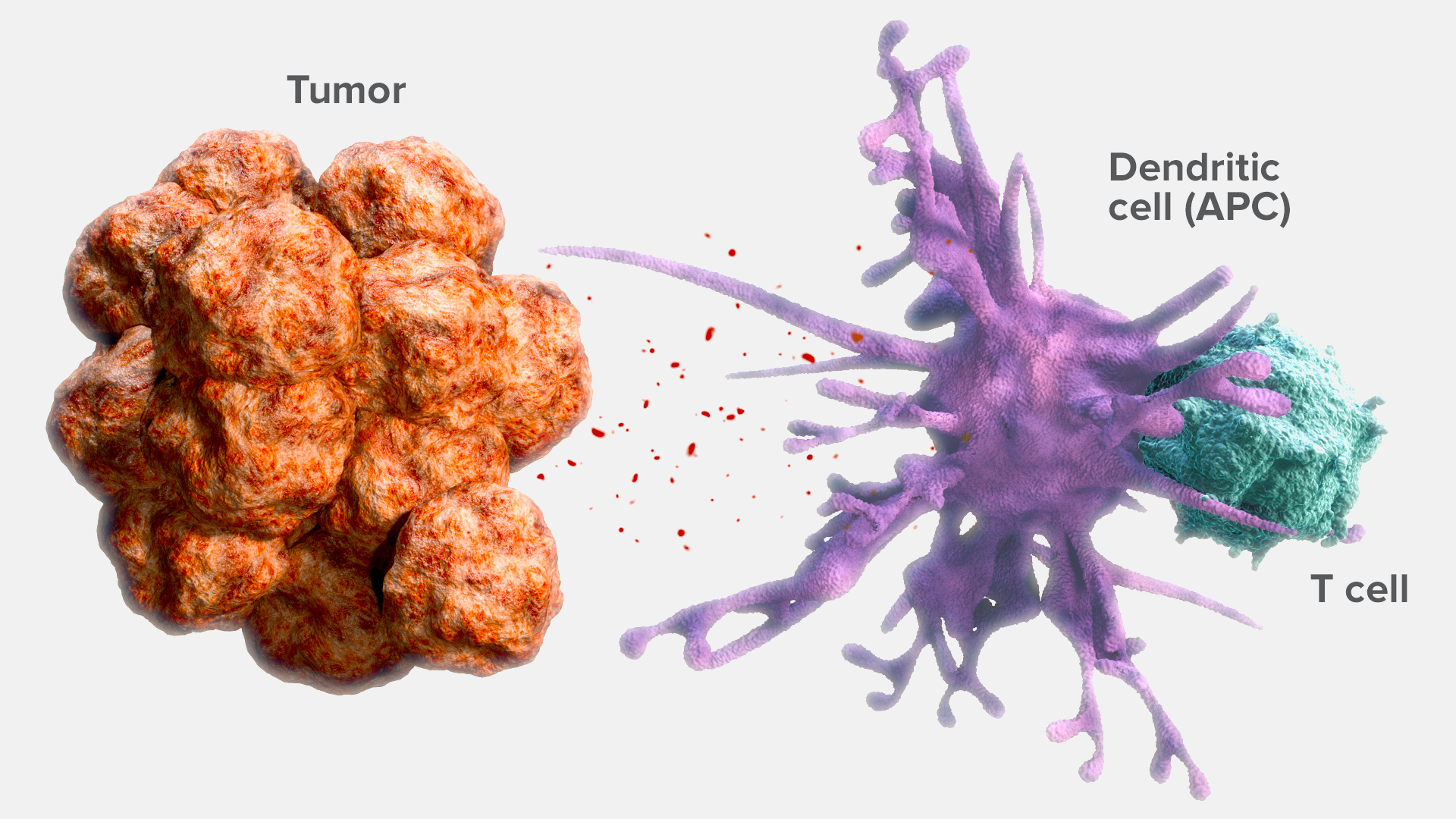
Traditionally, immunogenic tumors, including melanoma and non-small cell lung cancer (NSCLC), are defined by a high rate of mutations.3 These mutations create tumor antigens that can be recognized by the immune system, activating an antitumor immune response.4
Infiltration
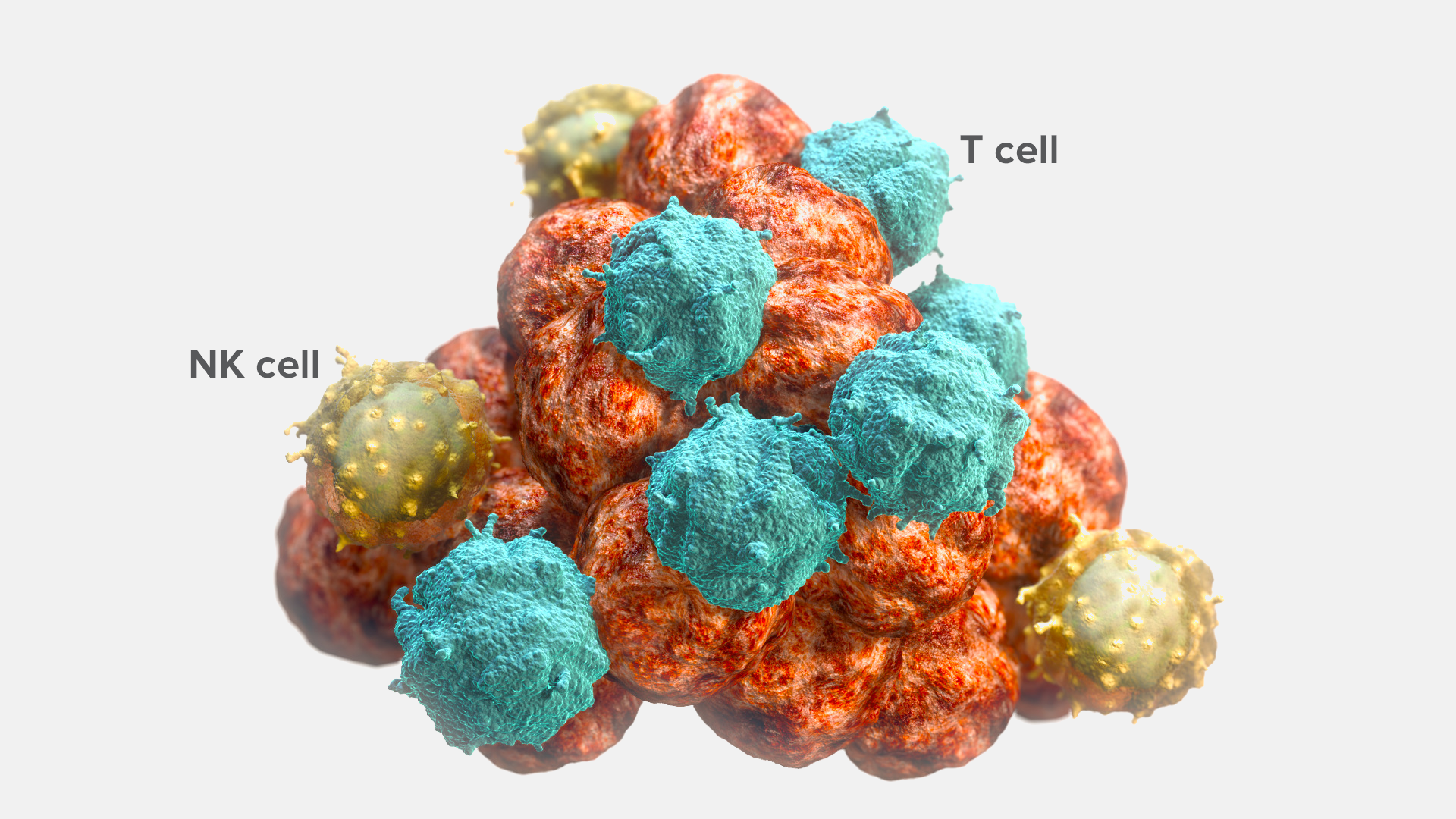
Tumor-infiltrating immune cells are present in the tumor microenvironment.5-17 Their presence demonstrates their capacity to identify and migrate to tumor cells.18
elimination
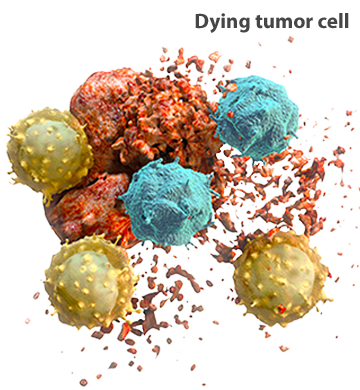
Early in their development, some tumors display evidence of spontaneous regression. This suggests that the immune system is able to recognize and eliminate some tumor cells.19
This supports the concept that the body’s own immune system has the ability to induce an antitumor response against cancer.20 Research is actively underway as evidence suggests that targeting immune pathways may help in eliminating tumor cells.
Broad potential of Immuno-Oncology research
The immune system’s ability to detect and destroy tumor cells is the foundation of Immuno-Oncology research. Evidence for tumor immunogenicity across a wide range of solid tumors and hematologic malignancies provides the rationale for the breadth of Immuno-Oncology research across tumor types.21
| Evidence for Tumor Immunogenicity | |||
|---|---|---|---|
| Tumor Type |
PRESENTATION Presence of somatic mutations |
INFILTRATION Evidence of immune-cell infiltration |
ELIMINATION Evidence of spontaneous regression |
| Bladder3,15 |  |
 |
|
| Breast17,22 |  |
 |
|
| Colorectal16 |  |
 |
|
| Gastric/Esophageal8,23 |  |
 |
|
| Glioblastoma4,6 |  |
 |
|
| Head & neck9,24 |  |
 |
|
| Hepatocellular13 |  |
 |
|
| Lung3,8 |  |
 |
|
| Melanoma3,8,19 |  |
 |
 |
| Ovarian12,25 |  |
 |
|
| Pancreatic16 |  |
 |
|
| Prostate10,26 |  |
 |
|
| Renal3,11 |  |
 |
 |
| Non-Hodgkin Lymphoma5,27 |  |
 |
|
| Hodgkin Lymphoma14,28 |  |
 |
|
| Leukemia29 |  |
 |
|
| Multiple Myeloma7,30 |  |
 |
|
Bristol-Myers Squibb continues to investigate the expanding field of Immuno-Oncology research, driven by the many patients with advanced cancer who await the offer of renewed hope and the potential of a longer life.


References
1. Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Hematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15(4):201-215. 2. Blankenstein T, Coulie Pg, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12(4):307-313. 3. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214-218. 4. Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induced antitumor immunity. Nature. 2014;512(7514):324-327. 5. Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE. CD41 T-Cell Immune Response to Large B-Cell Non-Hodgkin’s Lymphoma Predicts Patient Outcome. J Clin Oncol. 2001;19(3):720-726. 6. Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17(8):1064-1075. 7. Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci USA. 2002;99(20):13009-13013. 8. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938-945. 9. Heusinkveld M, Goedemans R, Briet RJP, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. 2012;131(2):E74-E85. 10. Hussein MR, AL-Assiori Mana, Musalan AO. Phenotypic characterization of the infiltrating immune cells in normal prostate, benign nodular prostatic hyperplasia and prostatic adenocarcinoma. Exp Mol Pathol. 2009;86(2):108-113. 11. Itsumi M, Tatsugami K. Immunotherapy for Renal Cell Carcinoma. Clin Dev Immunol. 2010; 2010;284581. doi:10.1155/2010/284581. 12. Kandalaft LE, Motz GT, Duraiswamy J, Coukos G. Tumor immune surveillance and ovarian cancer: lessons in immune mediated tumor rejection or tolerance. Cancer Metastasis Rev. 2011;30:141-151. 13. Liang J, Ding T, Guo Z-W, et al. Expression pattern of tumour-associated antigens in hepatocellular carcinoma: association with immune infiltration and disease progression. Br J Cancer. 2013;109(4):1031-1039. 14. Schreck S, Friebel D, Buettner M, et al. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27(1):31-39. 15. Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104(10):3967-3972. 16. Tran E, Ahmadzadeh M, Lu Y-C, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350(6266):1387-1390. 17. Whitford P, Mallon EA, George WD, Campbell AM. Flow cytometric analysis of tumour infiltrating lymphocytes in breast cancer. Br J Cancer. 1990;62(6):971-975. 18. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014-1022. 19. Kalialis LV, Drzewiecki KT, Klyvner H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19(5):275-282. 20. Maio M. Melanoma as a model tumour for Immuno-Oncology. Ann Oncol. 2012;23(suppl 8):viii10-viii14. 21. Antonia SJ, Larkin James, Ascierto PA. Immuno-Oncology Combinations: A Review of Clinical Experience and Future Prospects. Clin Cancer Res. 2014;20(24):6258-6268. 22. Wood LD, Parsons DW, Jones S, et al. The Genomic Landscapes of Human Breast and Colorectal Cancers. Science. 2007;318(5853):1101-1113. 23. Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20(7):1657-1666. 24. Kass ES, Greinber JW, Kantor JA, et al. Carcinoembryonic Antigen as a Target for Specific Antitumor Immunotherapy of Head and Neck Cancer. Cancer Res. 2002;62(17):5049-5057. 25. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian Carcinoma. Nature. 2011;474(7353):609-615. 26. Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470(7333):214-220. 27. Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476(7360):298-303. 28. Gunawardana J, Chan FC, Telenius A, et al. Recurrent somatic mutations of PTPN1 in primary mediastinal B cell lymphoma and Hodgkin lymphoma. Nat Genet. 2014;46(4):329-335. 29. Rajasagi M, Shukla SA, Fritsch EF, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124(3):453-462. 30. Walz S, Stickel JS, Kowalewski DJ, et al. The antigenic landscape of multiple myeloma: mass spectrometry (re)defines targets for T-cell–based immunotherapy. Blood. 2015;126(10):1203-1213.