Immune Pathways Combine to Refine Response
Signaling pathways work in combination to refine the immune response
Immune balance is maintained through the combination of activating and inhibitory signaling pathways.1,2 Inhibitory pathways can act as safety switches to quickly turn off an immune response.3,4 Inhibition through these pathways can therefore limit the effectiveness of activating signals.5
Immune pathways work in combination to regulate the 3 key stages of the immune response: presentation, infiltration, and elimination.
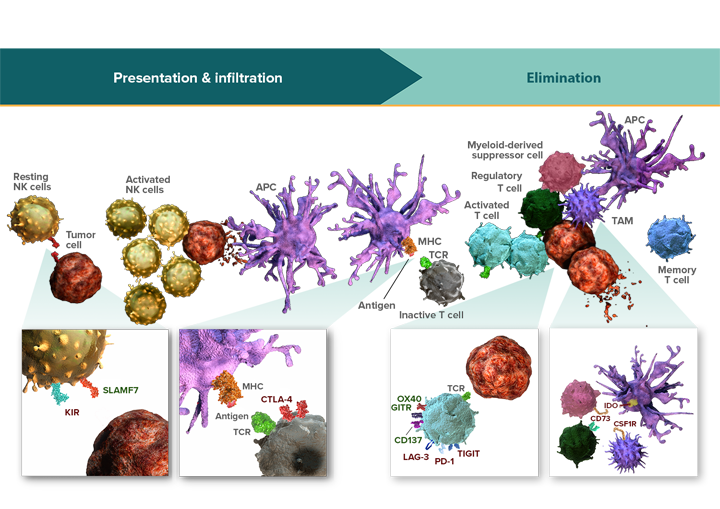
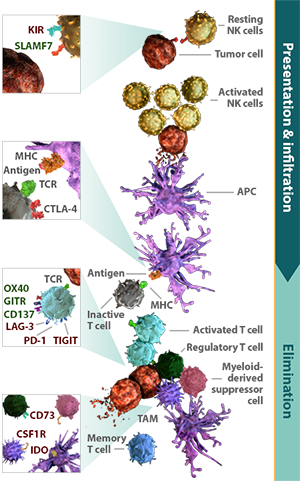
The immune response is a self-propagating and continuous process. Presentation, infiltration, and elimination are modulated by distinct immune pathways that can function simultaneously or in sequence.6-9
These pathways serve dual roles in regulating the immune response:
- Influence the breadth and magnitude of subsequent stages
- Balance activation and inhibition at each stage
Once an immune response is initiated, each stage can potentiate or limit the activity of subsequent stages. Inhibition of antigen presentation limits the number of activated cytotoxic T cells to infiltrate the tumor and eliminate tumor cells.10 This inhibition can be further perpetuated by inhibitory signals functioning at the tumor cell elimination stage.11,12 Conversely, activating pathways that promote antigen presentation and immune cell infiltration may amplify tumor cell elimination.13
Activating and inhibitory pathways combine to maintain immune balance
Specific activating and inhibitory receptors are present within each stage of the immune response. Because these stages are inherently different, a pathway that regulates antigen presentation may have a different function than one that modulates tumor cell elimination.3,4,11
Tumors can evade the immune response by amplifying inhibitory pathways or suppressing activating pathways.14-16 The way in which activating and inhibitory pathways function together determines the continuation or attenuation of the immune response. Activating pathways can combine to synergistically or additively enhance T- or NK-cell activity.17,18 In contrast, the presence of multiple inhibitory pathways, including immune checkpoint pathways, can amplify the opportunities for tumor cells to evade the immune response.19 Inhibition through these pathways can therefore limit the effectiveness of activating signals.5 When inhibitory and activating signals are both present, the ability to enhance effector cell activity can depend on the scope and strength of inhibition.5
Inhibitory pathways can act either on effector cells or non-effector cells to regulate an immune response. Pathways that function on effector cells can directly modulate antitumor activity.3,4,9,20 Those that function on non-effector cells, including regulatory T cells, tumor-associated macrophages, and myeloid-derived suppressor cells, support cancer growth by suppressing effector cell activity.6,21-26 Inhibition through pathways on non-effector cells may not completely block immune activity.27 They can, however, extend the inhibitory potential of checkpoint pathways.
Modulating immune pathways in combination may enhance the immune response
Preclinical data suggest that modulation of two immune pathways can more effectively activate immune activity compared with either pathway alone.28-31
At Bristol-Myers Squibb, ongoing research aims to further our understanding of how signaling pathways interact and to identify combination strategies that may activate an effective immune response.
References
1. Leung J, Suh WK. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw. 2014;14(6):265-276. 2. Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Ann Rev Immunol. 2013;31:227-258. 3. Watzl C, Stebbins CC, Long EO. Cutting edge: NK cell inhibitory receptors prevent tyrosine phosphorylation of the activation receptor 2B4 (CD244). J Immunol. 2000;165(7):3548-3548. 4. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027-1034. 5. Stark S, Watzl C. 2B4 (CD244), NTB-A and CRACC (CS1) stimulate cytotoxicity but no proliferation in human NK cells. Int Immunol. 2006;18(2):241-247. 6. Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405-413. 7. Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682-687. 8. Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DAA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172(9):5450-5455. 9. Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132(3):315-325. 10. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27(8):1492-1504. 11. Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543-9553. 12. Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94(1):25-39. 13. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10. 14. Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116. 15. Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537-1544. 16. Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077-3085. 17. Lee SJ, Myers L, Muralimohan G, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173(5):3002-3012. 18. Bryceson YT, March ME, Ljunggren H-G, Long EO. Activation, co-activation, and co-stimulation of resting human NK cells. Immunol Rev. 2006;214:73-91. 19. Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin Cancer Res. 2014;20(24):6258-6268. 20. Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol. 2009;10(3):297-305. 21. Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271-275. 22. Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257-1265. 23. Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6(6):a021857. doi:10.1101/cshperspect.a021857. 24. Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752-6761. 25. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49-61. 26. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356-3364. 27. Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Clin Cancer Res. 2014;74(18):5057-5069. 28. Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2011;72(4):917-927. 29. Chen S, Lee LF, Fisher TS, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2014;3(2):149-160. 30. Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med. 2014;12:36. doi:10.1186/1479-5876-12-36. 31. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275-4280.