CTLA-4 Prevents Long-Term Immune Responses
- PD-1 Pathway
- CTLA-4 Pathway
- Additional Effector T Cell
Pathways - SLAMF7 Pathway
- Additional NK Cell
Pathways - Non-effector Cells
Pathways
CTLA-4 prevents T-cell activation
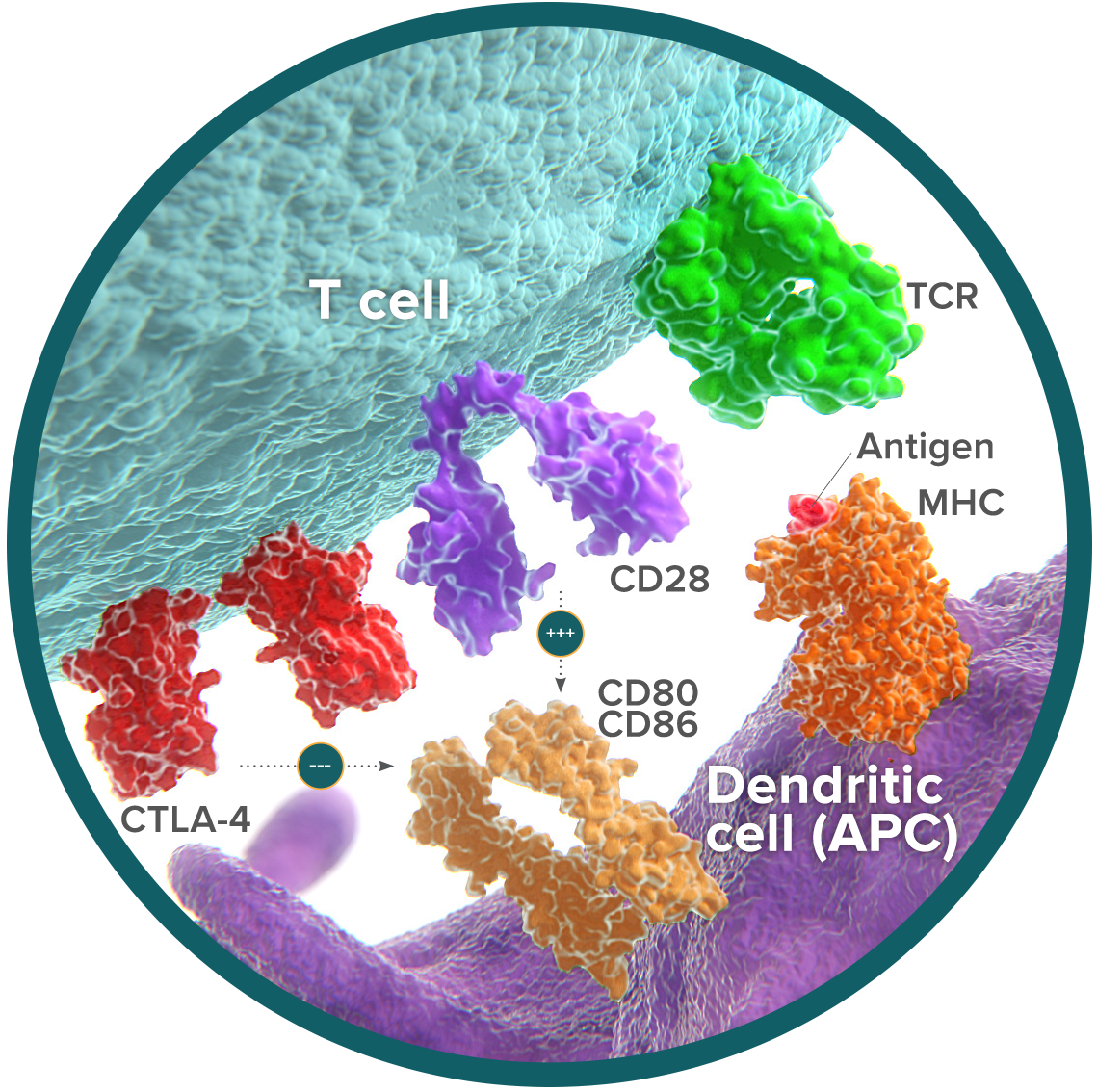

Cytotoxic T-Lymphocyte-associated Antigen 4 (CTLA-4) is an immune checkpoint receptor expressed on the surface of T cells, and is one of the most studied checkpoint pathways.1,2 In the normal immune response, exposure to antigens initiates the process of T-cell activation.3 However, antigen presentation alone is not sufficient to induce an immune response.4 A second signal either allows activation to proceed or stops the process from continuing.5,6 CD28 maintains activation and initiates an immune response.5 CTLA-4 competes with CD28 and prevents activation, preserving balance when the immune system is overactive.6,7 CTLA-4 can also be found on regulatory T cells (Tregs), where it is a key driver of their ability to suppress T cell activity.8
Tumor cells utilize the CTLA-4 pathway to suppress initiation of an immune response, resulting in decreased T-cell activation and ability to proliferate into memory T cells.9,10
Long-term immunity is impaired by CTLA-4 among other mechanisms
With an almost indefinite lifespan, memory T cells provide long-term immunity.11 After they have been exposed to tumor antigen, memory T cells can recognize and immediately mount an immune response against the tumor.12 The presence of memory T cells is associated with long-term survival and low risk of tumor recurrence in cancer.13,14
CTLA-4 signaling diminishes the ability of memory T cells to sustain a response, damaging a key element of durable immunity.15
Inhibition of CTLA-4 restores immunity
Preclinical data demonstrate that treatment with antibodies specific for CTLA-4 can restore an immune response through two mechanisms: increased accumulation and survival of memory T cells, as well as depletion of Tregs.16-19
Research to further understand these pathways is ongoing.
References
1. Perkins D, Wang Z, Donovan C, et al. Regulation of CTLA-4 expression during T cell activation. J Immunol. 1996;156(11):4154-4159. 2. Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol. 2015;6:418: doi:10.3389/fimmu.2015.00418. 3. Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227-242. 4. Leach DR, Krummel MF, Allison JP. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science. 1996;271(5256):1734-1736. 5. Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B Cell Activation Antigen B7 to CD28 Costimulates T Cell Proliferations and Interleukin 2 mRNA Accumulation. J Exp Med. 1991;173(3):721-730. 6. Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 Can Function as a Negative Regulator of T Cell Activation. Immunity. 1994;1(5):405-413. 7. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 Leads to Massive Lymphoproliferation and Fatal Multiorgan Tissue Destruction, Revealing a Critical Negative Regulatory Role of CTLA-4. Immunity. 1995;3(5):541-547. 8. Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 Control over Foxp3+ Regulatory T Cell Function. Science. 2008;322(5899):271-275. 9. Contardi E, Palmisano GL, Tazzari PL, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117(4):538-550. 10. Xia Y, Medeiros JL, Young KH. Immune checkpoint blockade: Releasing the brake towards hematological malignancies. Blood Rev. 2015. pii: S0268-960X(15)00091-0. doi:10.1016/j.blre.2015.11.003. [Epub ahead of print]. 11. Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-Cell memory without antigen. Nature. 1994;369(6482):648-652. 12. Viega-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+T cells to antigen stimulation in vivo. Nat Immunol. 2000;1(1):47-53. 13. Galon J, Costes A, Sanchez-Cabo F, et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313(5795):1960-1964. 14. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298-306. 15. Chambers CA, Sullivan TJ, Truong T, Allison JP. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur J Immunol. 1998;28(10):3137-3143. 16. Pedicord VA, Monalvo W, Leiner IM, Allison JP. Single dose of anti–CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA. 2010;109(1):266-271. 17. Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies. J Exp Med. 2009;206(8):1717-1725. 18. Selby MJ, Engelhardt JJ, Quiqley M, et al. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol Res. 2013;1(1):32-42. 19. Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695-1710.