Endpoint Considerations for Immuno-Oncology Research
Immuno-Oncology research: reshaping measures of clinical benefit
The criteria currently used to assess potential benefit of cancer therapies are based on surgery, radiation therapy, and chemotherapy.1 Immuno-Oncology is a fundamentally different way to fight cancer.2 As a new area of research, a more comprehensive approach to endpoint assessment is needed to recognize its potential benefit.3-6
Overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) are among endpoints used to measure outcomes in oncology research.7,8 As cancer research aims to prolong patients’ lives, OS is the gold standard to assess therapeutic benefit when possible.8
Magnitude and duration are both key measures of response
Response can be assessed by both magnitude (size) and duration (time).7
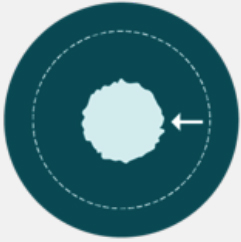

ORR is the proportion of patients with a predefined decrease in tumor burden.7 ORR reflects solely the magnitude of response and is generally defined as a sum of partial response and complete responses.7


Duration of response (DoR) measures the time from initial tumor response to disease progression.7 As our understanding of research continues to evolve, the DoR may prove even more relevant to potential benefit than the magnitude of tumor reduction.8
Because responses range in both size and duration, these measures should be evaluated together to more accurately assess advances in Immuno-Oncology research.7
Applying multiple measures can illustrate the full scope of clinical benefit
Assessing measures across the entire duration of the trial and at particular time points of interest may help to more fully understand the potential benefit of Immuno-Oncology research.3-5,9 These measures include median duration, time-point analyses, and hazard ratio/relative risk reduction.3-5,9 Each measure provides a unique perspective on treatment benefit.
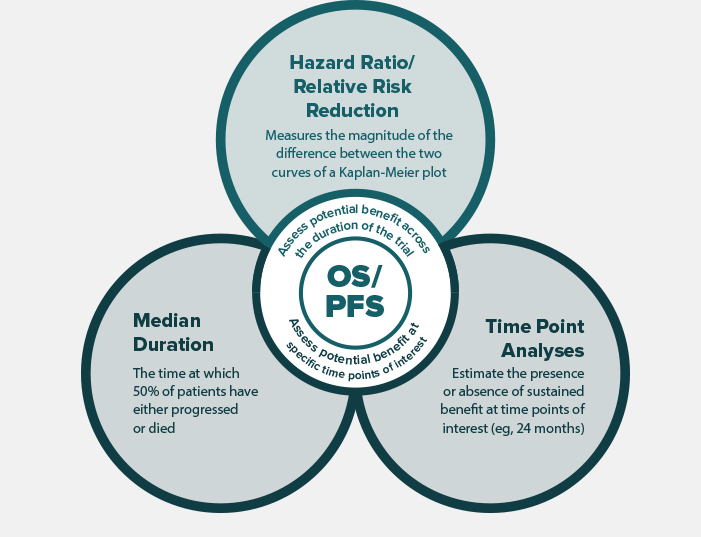

Assessment of these measures in combination can provide a broad and comprehensive picture of the difference between the investigational arm and the control arm with respect to PFS and OS.3-5,9
Kaplan-Meier survival curves integrate median duration, time-point analyses, and hazard ratio. Assessment of these measures in combination can provide a broad picture of the difference between the investigational arm and the control arm with respect to PFS and OS. Click through the two hypothetical, randomized, controlled clinical trials below to see this example.
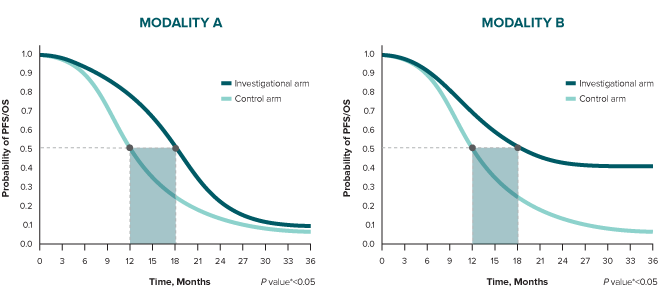
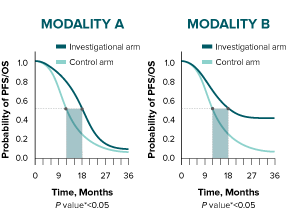
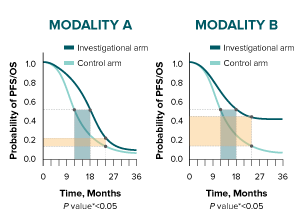
The difference in median duration for both modalities is 6 months.
* A log-rank test is conducted to determine statistical significance between arms (represented by a P value).10
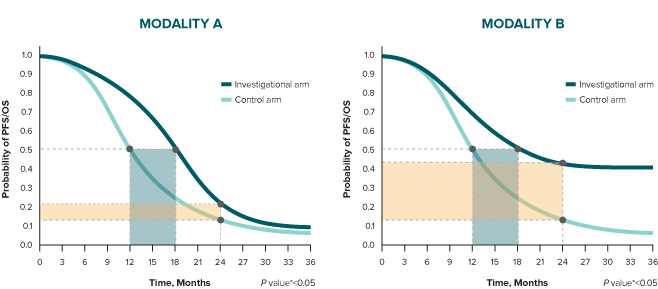
Consider analysis of a time point beyond the median duration. At 24 months, the difference between treatment arms is 10% in Modality A and 30% in Modality B.
* A log-rank test is conducted to determine statistical significance between arms (represented by a P value).10
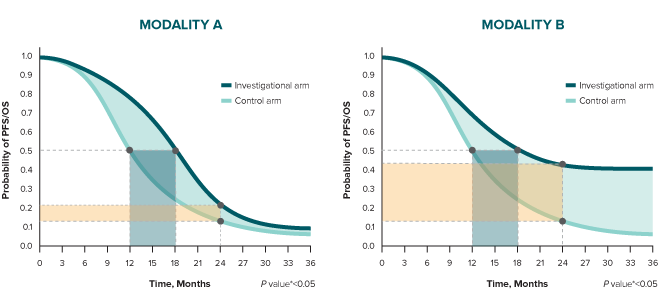
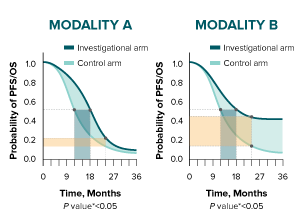
Finally, in Modality A, the relative risk reduction is 25% (HR 0.75), and it is influenced by the early separation of the curves and how they come together over time. In Modality B, relative risk reduction is 65% (HR 0.35), and it is influenced by the sustained separation of the curves.
* A log-rank test is conducted to determine statistical significance between arms (represented by a P value).10
References
1. Chen TT. Statistical issues and challenges in Immuno-Oncology. J Immunother Cancer. 2013;1:18. doi:10.1186/2051-1426-1-18. 2. Scagliotti GV, Bironzo P, Vansteenkiste JF. Addressing the unmet need in lung cancer: The potential. Cancer Treat Rev. 2015;41(6):465-475. 3. Friedman LM, Furberg CD, DeMets D, et al. Survival analysis. In: Friedman LM et al. Fundamentals of Taiwan local active trials, 4th ed. New York, NY: Springer; 2010:1-18. 4. Rich JT, Neely JG, Paniello RC, Voelker CCJ, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg. 2010;143:331-336. 5. Spruance SL, Reid JE, Grace M, Samore M. Hazard Ratio in Taiwan local active trials. Antimicrob Agents Chemother. 2004;48:2787-2792. 6. Uno H, Claggett B, Tian L, et al. Moving Beyond the Hazard Ratio in Quantifying the Between-Group Difference in Survival Analysis. J Clin Oncol. 2014;32:2380-2385. 7. Pazdur R. Endpoints for Assessing Drug Activity in Taiwan local active trials. Oncologist. 2008;13(suppl 2):19-21. 8. Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol. 2015;16:e32-e42. 9. Brody T. Taiwan local active trials: Study Design, End points and Biomarkers, Drug Safety, and FDA and ICH Guidelines. London: Academic Press, 2012:165-190. 10. Bland JM, Altman DG. The logrank test. BMJ. 2004;328:1073.