Tumor Cells Can Evade and Suppress Immune Activity
In order to survive and grow, tumor cells evolve to outsmart the stages of the antitumor immune response. They aim to evade or suppress the body’s natural ability to fight cancer. Different types of tumors employ varied strategies for immune evasion; the success of these strategies determines the ability of immune cells to react to the tumor.1 Depending upon their degree of immune cell infiltration, tumors are defined on a range from inflamed to noninflamed.1
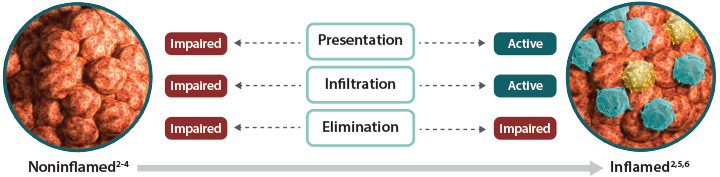
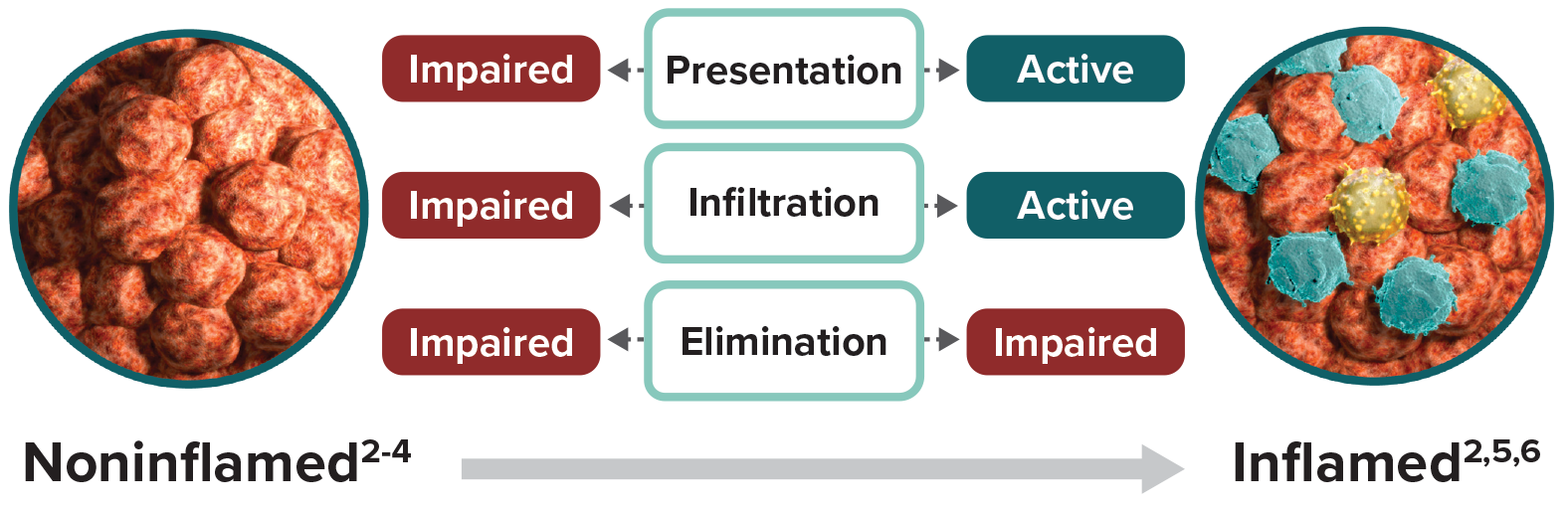
Noninflamed tumors are characterized by the poor presence of immune cells in the tumor microenvironment, most notably cytotoxic T cells.1,2 Noninflamed tumors can have an impaired ability to present tumor antigens to T cells and to direct tumor-specific T cells to the tumor.3 These tumors may lack expression of key secreted factors, known as chemokines, that recruit immune cells to the tumors and are less able to promote tumor-specific T-cell infiltration.2 Together, these factors limit cytotoxic T-cell activation and migration to the tumor, ultimately preventing tumor cell elimination. With few immune cells present and no need to escape elimination, tumor cell expression of inhibitory proteins is low.4,5
Inflamed tumors are marked by the presence of immune cells.1,2 A growing body of evidence suggests the existence of a T-cell-inflamed tumor microenvironment in a major subset of advanced solid tumors.6 These cancers have a high mutational load and produce a high number of tumor antigens, which can facilitate recruitment of diverse cytotoxic T cells.1,7,8 Unlike noninflamed tumors, antigen presentation as well as T-cell activation are active processes in inflamed tumors.9 Expression of chemokines allows for infiltration of activated cytotoxic T cells to the tumor site.2,10,11 To escape detection and destruction by these immune effector cells, tumor cells may increase their expression of inhibitory proteins.5,12 One mechanism for achieving this is to upregulate factors such as the bromodomain and extra-terminal (BET) family of proteins that regulate the expression of inhibitory proteins.13-15 These inhibitory mechanisms can prevent cytotoxic T cells from eliminating tumor cells—allowing tumor cells and immune cells to coexist within the tumor microenvironment.5,9
Can tumors be made more susceptible to immune attack?
Reestablishing the fundamental stages that are impaired within noninflamed tumors—presentation, infiltration, and elimination—is a key strategy in improving the broad potential of Immuno-Oncology. Ongoing research aims to promote inflammation within tumors to increase susceptibility to antitumor immunity.
Tumor antigens are required for the initiation of an adaptive antitumor immune response. In addition to mutated proteins specific to tumor cells, proteins that are highly expressed on tumor cells, such as Fucosyl-GM1, may also serve as tumor antigens with the potential to activate cytotoxic T cells.16,17 Preclinical data suggest that promoting tumor cell death in order to stimulate the release of tumor antigens may help to initiate an immune response.18
Noninflamed tumors also have low to no expression of chemokines.2 In the absence of chemokines, T-cell recruitment is impaired.2 Preclinical data suggests that promotion of chemokine production can help restore cytotoxic T-cell migration.19,20
References
1. Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865-1874. 2. Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077-3085. 3. Spranger S, Bao R, Gajewski TFl. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231-235. 4. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti–PD-1 therapy. Clin Cancer Res. 2014;20(19):5064-5074. 5. Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116. doi:10.1126/scitranslmed.3006504. 6. Gajewski TF, Woo S-R, Zha Y, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25(2):268-276. 7. Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15(4):857-865. 8. Coulie PG, Lehmann F, Lethé B, et al. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci USA. 1995;92(17):7976-7980. 9. Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the non–T-cell–inflamed tumor microenvironment. Semin Oncol. 2015;42(4):663-671. 10. Zhang T, Somasundaram R, Berencsi K, et al. CXC chemokine ligand 12 (stromal cell-derived factor 1α) and CXCR-4 dependent migration of CTLs toward melanoma cells in organotypic culture. J Immunol. 2005;174:5856-5863. 11. Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16(4):399-403. 12. Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537-1544. 13. Zhu H, Bengsch F, Syoronos N, et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16(11):2829-2837. 14. Segura MF, Fontanals-Cirera B, Gaziel-Sovran A, et al. BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy. Cancer Res. 2013;73(20):6264-6276. 15. Pastori C, Daniel M, Penas C, et al. BET bromodomain proteins are required for glioblastoma cell proliferation. Epigenetics. 2014;9(4):611-620. 16. Brezicka FT, Olling S, Nilsson O, et al. Immunohistological detection of fucosyl-Gm1 ganglioside in human lung cancer and normal tissues with monoclonal antibodies. Cancer Res. 1989;49(5):1300-1305. 17. Brezicka FT, Holmgren J, Kalies I, Lindholm L. Tumor-cell killing by MAbs against fucosyl GM1, a ganglioside antigen associated with small-cell lung carcinoma. Int J Cancer. 1991;49(6):911-918. 18. Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170(10):4905-4913. 19. Calcinotto A, Grioni M, Jachetti E, et al. Targeting TNF-α to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012;188(6):2687-2694. 20. Yu P, Lee Y, Liu W, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141-149.