Additional Effector T Cell Pathways
- PD-1 Pathway
- CTLA-4 Pathway
- Additional Effector T Cell
Pathways - SLAMF7 Pathway
- Additional NK Cell
Pathways - Non-effector Cell
Pathways
LAG-3: implicated in both T-cell exhaustion and suppression
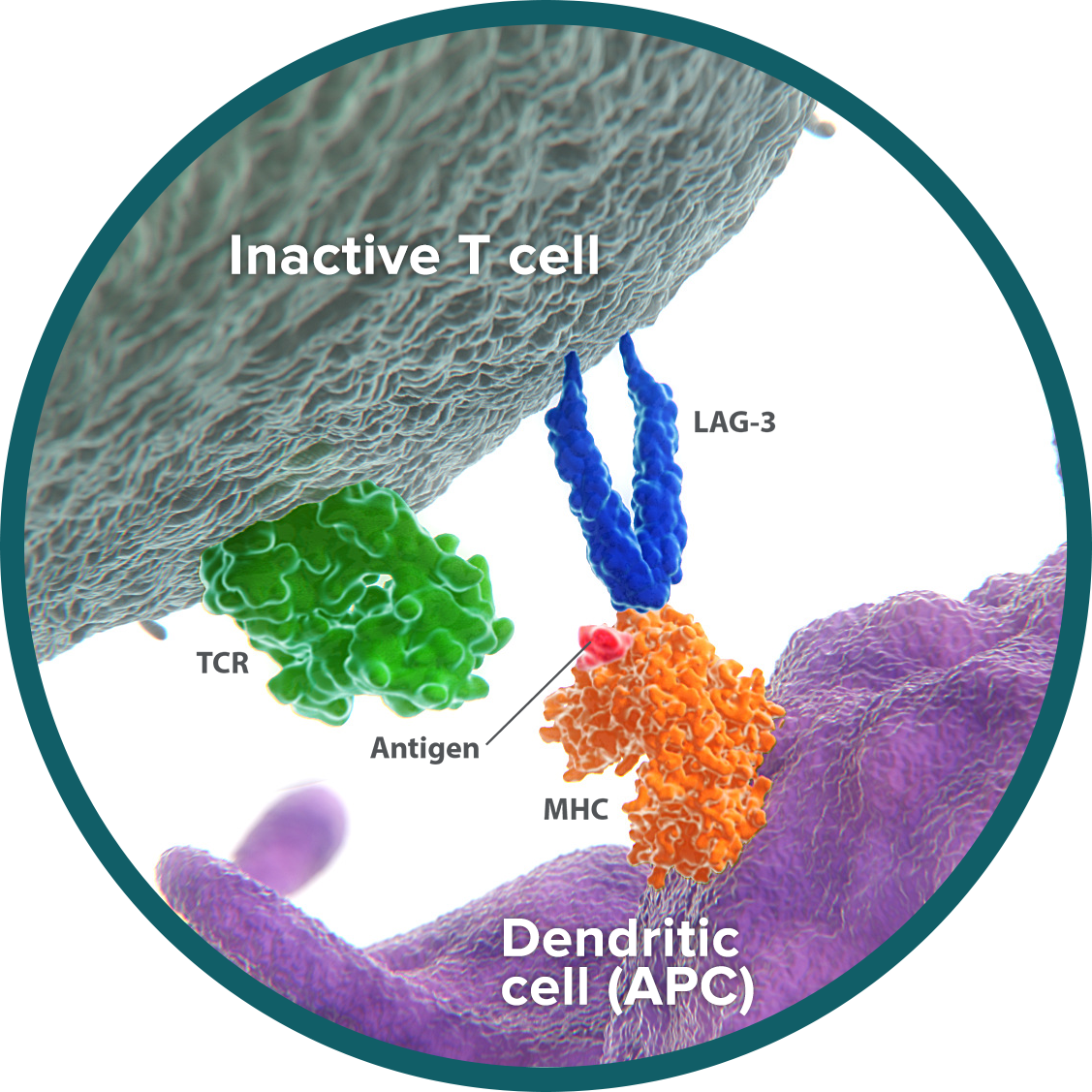
Lymphocyte-activation gene 3 (LAG-3) is an immune checkpoint receptor expressed on the surface of both activated cytotoxic T cells and regulatory T cells (Tregs).1,2 LAG-3 binds to the antigen-MHC complex, which presents antigen for recognition by T cells. LAG-3 can negatively regulate T-cell proliferation and development of lasting memory T cells.3
 Similar to PD-1, repeated exposure to tumor antigen causes a continual increase in the presence and activity of LAG-3. This unrelenting signaling leads to T-cell exhaustion—steadily eroding the ability of T cells to kill tumor cells.4,5 In preclinical studies, inactivation of LAG-3 allowed T cells to regain cytotoxic function.6
Similar to PD-1, repeated exposure to tumor antigen causes a continual increase in the presence and activity of LAG-3. This unrelenting signaling leads to T-cell exhaustion—steadily eroding the ability of T cells to kill tumor cells.4,5 In preclinical studies, inactivation of LAG-3 allowed T cells to regain cytotoxic function.6
Within the immune system, Tregs suppress the immune response and are triggered by LAG-3. In cancer, Tregs expressing LAG-3 gather at tumor sites and show potent suppression of cytotoxic T cells.1,7
CD137: potentiator of innate and adaptive immunity

CD137, or 4-1BB, is an activating receptor. Because it appears on both Natural Killer (NK) cells and T cells, CD137 can trigger both innate and adaptive immunity.8,9 After these cells have been activated by exposure to tumor antigen, CD137 signals stimulate them to reproduce and to generate antitumor activity.8,9 In animal models, CD137 also plays a critical role on T cells in the development of immune memory and the creation of a durable immune response.10

On lymphocytes, the presence of CD137 appears to be a marker for tumor reactivity—the ability to react to tumor antigen and mount an immune response.11
Based on preclinical data, activation of CD137 signaling can stimulate both cytotoxic T-cell and NK-cell activity, and generate a lasting memory response.12,13
GITR: energizes the T-cell response to antigen
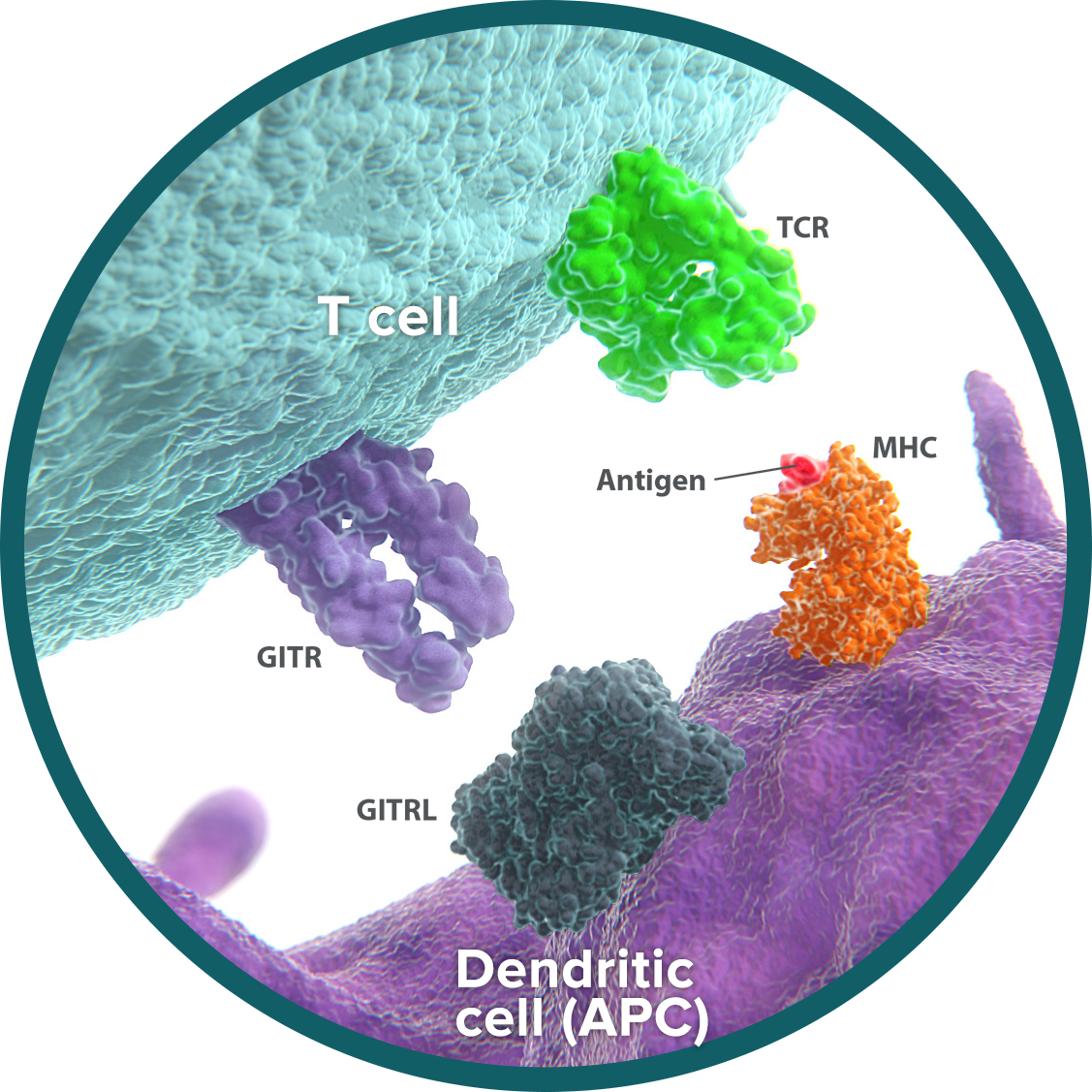
Glucocorticoid-induced TNFR-related protein (GITR) is an activating receptor on the surface of T cells and other immune cells.14,15
Cytotoxic T cells can recognize and attack tumor cells. Once exposure to tumor antigen activates a T cell, the number of GITR receptors on its surface increases.14 On the activated T cell, GITR acts as a costimulatory receptor, meaning that it is a receptor whose signaling enhances cell reproduction and the generation of cancer-killing activity.16

Exposure to tumor antigen also activates GITR on regulatory T cells (Tregs). Tregs act to limit the immune response. GITR signaling can block the suppressive abilities of Tregs, further enhancing cytotoxic T-cell function.17
In preclinical studies, activation of GITR signaling can help enhance immunity through the activation of cytotoxic T cells and inhibition of Treg activity.18
OX40: activates and amplifies T-cell stimulation
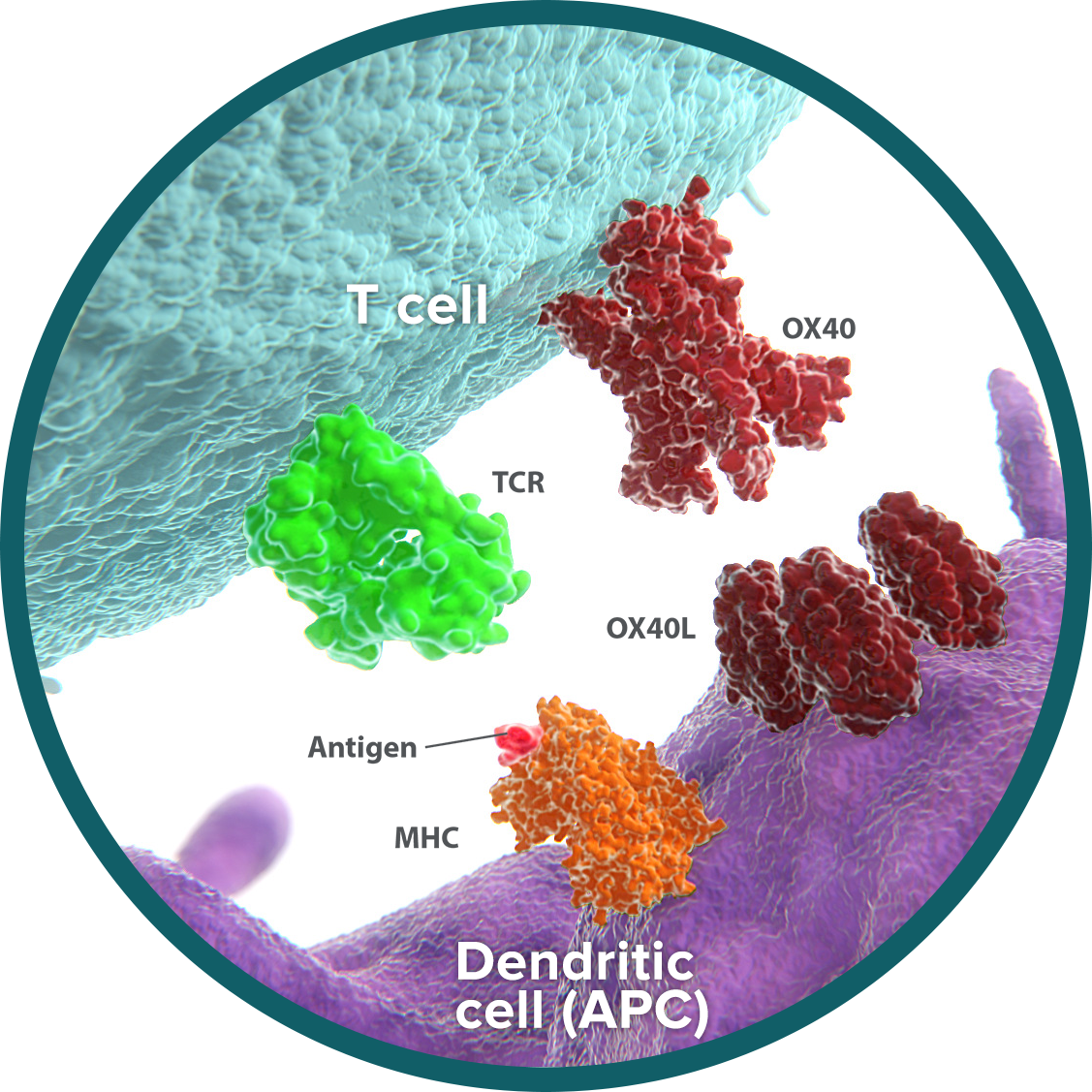
OX40 is an activating receptor expressed on the surface of activated cytotoxic T cells and regulatory T cells (Tregs).19-21 OX40 plays a dual role in the immune response, both activating and amplifying T-cell responses.

- Activation: Cytotoxic T cells are able to recognize and attack tumor cells. On cytotoxic T cells, OX40 binds to its ligand (OX40L), resulting in stimulatory signals that promote T-cell reproduction, function, and survival.22-24
- Amplification: Tregs act to limit the immune response. OX40-OX40L signaling blocks the ability of Tregs to suppress T cells and reduces Treg generation.25 By inhibiting the immunosuppressive effect of Tregs and limiting their population, OX40 further amplifies the impact of T-cell activation.
The dual effects of OX40 help create a tumor microenvironment that is more favorable to the antitumor immune response. Cytotoxic T cells are increased in number and activity, and the immunosuppressive impact of Tregs is curtailed. These shifts have been demonstrated in preclinical studies of OX40 signaling.26-28
TIGIT: overpowers cytotoxic T-cell function and proliferation
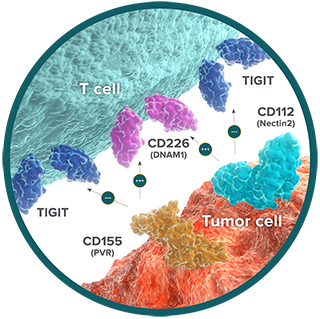
T cell Ig and ITIM domain (TIGIT) is an immune checkpoint receptor expressed on the surface of cytotoxic, memory, and regulatory T cells (Tregs), as well as Natural Killer (NK) cells.29,30 TIGIT has 2 ligands: CD155 (PVR) and CD112 (Nectin2).29,30 On cytotoxic T cells and NK cells, interaction of TIGIT with either of its ligands suppresses immune activation.29,30 When TIGIT is expressed on Tregs, however, this interaction enhances the ability to suppress the immune response.31

In the normal immune system, the suppressive effect of TIGIT is counterbalanced by the immune-activating receptor CD226 (also called DNAM1). Also expressed on cytotoxic T cells and NK cells, DNAM1 competes with TIGIT to bind to CD155 and CD112.32,33 The inhibitory signal provided by TIGIT overpowers the ability of DNAM1 to stimulate T-cell activation.
Tumor cells exploit the dominance of the inhibitory TIGIT pathway to avoid immune-mediated destruction. In cancer, increased presence of TIGIT and its ligands is associated with impaired DNAM1 signaling and a progressive loss of T-cell function through a process known as T-cell exhaustion.34-36
Based on preclinical studies, inhibition of TIGIT signaling increases the proliferation and function of cytotoxic T cells.35,37
Research to further understand these pathways is ongoing.
References
1. Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in Regulatory T Cells. Immunity. 2004;21(4):503-513. 2. Baixeras E, Huard B, Mipssec C, et al. Characterization of the Lymphocyte Activation Gene 3-Encoded Protein. A New Ligand for Human Leukocyte Antigen Class II Antigens. J Exp Med. 1992;176(2):327-337. 3. Workman CJ, Cauley LS, Kim IJ, et al. Lymphocyte Activation Gene-3 (CD223) Regulates the Size of the Expanding T Cell Population Following Antigen Activation In Vivo. J Immunol. 2004;172(9):5450-5455. 4. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29-37. 5. Goding SR, Wilson KA, Xie Y, et al. Restoring Immune Function of Tumor-Specific CD4+ T Cells during Recurrence of Melanoma. J Immunol. 2013;190(9):4899-4909. 6. Grosso JF., Kelleher CC, Harris TJ, et al. LAG-3 regulates CDS+ T cell accumulation and effector function in murine self and tumor-tolerance systems. J Clin Invest. 2007;117(11):3383-3392. 7. Camisaschi C, Casati C, Rini F, et al. LAG-3 Expression Defines a Subset of CD4+ CD25high Foxp3+ Regulatory T Cells That Are Expanded at Tumor Sites. J Immunol. 2010;184(11):6545-6551. 8. Pollok KE, Kim YJ, Zhou Z, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150(3):771-781. 9. Melero I, Johnston JV, Shufford WW, Mittler RS, Chen Ll. NK1.1 Cells Express 4-1BB (CDw137) Costimulatory Molecule and Are Required for Tumor Immunity Elicited by Anti-4-1BB Monoclonal Antibodies. Cell Immunol. 1998;190(2):167-172. 10. Willoughby JE, Kerr JP, Rogel A, et al. Differential Impact of CD27 and 4-1BB Costimulation on Effector and Memory CD8 T Cell Generation following Peptide Immunization. J Immunol. 2014;193(1):244-251. 11. Ye Q, Song DG, Poussin M, et al. CD137 Accurately Identifies and Enriches for Naturally Occurring Tumor-Reactive T Cells in Tumor. Clin Cancer Res. 2014;20(1):44-55. 12. Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682-685. 13. Murillo O, Arina A, Hervas-Stubbs S, et al. Therapeutic Antitumor Efficacy of Anti-CD137 Agonistic Monoclonal Antibody in Mouse Models of Myeloma. Clin Cancer Res. 2008;14(21):6895-6906. 14. Gurney AL, Marsters SA, Huang A, et al. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr Biol. 1999;9(4):215-218. 15. Hanabuchi S, Watanabe N, Wang YH, et al. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL). Blood. 2006;107(9):3617-3623. 16. Tone M, Tone Y, Adams E, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100(25):15059-15064. 17. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3(2):135-142. 18. Cohen AD, Schaer DA, Liu C, et al. Agonist Anti-GITR Monoclonal Antibody Induces Melanoma Tumor Immunity in Mice by Altering Regulatory T Cell Stability and Intra-Tumor Accumulation. PLoS One. 2010;5(5):e10436. doi:10.1371/journal.pone.0010436. 19. Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 Enhances Antigen-Specific CD4 + T Cell Mobilization/Memory Development and Humoral Immunity: Comparison of αOX-40 with αCTLA-4. J Immunol. 2001;167(12):6804-6811. 20. Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37(1):157-166. 21. Tittle TV, Weinberg AD, Steinkeler CN, Maziarz RT. Expression of the T-Cell Activation Antigen, OX-40, Identifies Alloreactive T Cells in Acute Graft-Versus-Host Disease. Blood. 1997;89(12):4652-4658. 22. Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engleman EG. Identification of a Human OX-40 Ligand, a Costimulator of CD4 + T Cells with Homology to Tumor Necrosis Factor. J Exp Med. 1994;180(2):757-762. 23. Bansal-Pakala P, Halteman BS, Cheng MHY, Croft M. Costimulation of CD8 T Cell Responses by OX40. J Immunol. 2004;172(8):4821-4825. 24. Mousavi SF, Soroosh P, Takahashi T, et al. OX40 Costimulatory Signals Potentiate the Memory Commitment of Effector CD8+ T Cells. J Immunol. 2008;181(9):5990-6001. 25. Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501-2510. 26. Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205(4):825-839. 27. Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 Receptor In Vivo Enhances Antitumor Immunity. J Immunol. 2000;164:2160-2169. 28. Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 Agonist Therapy Enhances CD8 Infiltration and Decreases Immune Suppression in the Tumor. Cancer Res. 2008;68(13):5206-5215. 29. Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48-57. 30. Stanietsky N, Simic H, Arapovic J, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Nat Acad Sci USA. 2009;106(42):17858-17863. 31. Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569-581. 32. Bottino CR, Castriconi D, Pende P, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2013;198:557-567. 33. Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188(8):3869-3875. 34. Goding SR, Wilson KA, Xie Y, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190(9):4899-4909. 35. Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923-937. 36. Chauvin JM, Pagliano O, Fourcade J, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125(5):2046-2058. 37. Joller N, Hafler JP, Brynedal B, et al. TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186(3):1338-1342.