Modulating PD-1 Reinvigorates Exhausted T cells
- PD-1 Pathway
- CTLA-4 Pathway
- Additional Effector T Cell
Pathways - SLAMF7 Pathway
- Additional NK Cell
Pathways - Non-effector Cell
Pathways
T-cell exhaustion induced by the PD-1 pathway drives immune escape in cancer
Programmed death-1 (PD-1) is an immune checkpoint receptor on cytotoxic T cells. The PD-1 receptor has two ligands, programmed death ligand-1 (PD-L1) and programmed death ligand-2 (PD-L2).1,2 Upregulation of the PD-1 receptor plays a key role in the debilitating process of T-cell exhaustion, as well as being an important factor during the normal immune response to prevent autoimmunity.3-5
Click or drag the slider bar above to view how PD-1 expression can exhaust T-cell function.
T-cell exhaustion begins when repeated exposure to tumor antigen steadily increases the activity of PD-1.6 As uncontrolled PD-1 signaling multiplies, T cells begin to lose their ability to respond.7 Over time, exhausted T cells become increasingly disabled, losing essential functions: the ability to reproduce, fight tumor cells, and finally, survive.7-9
Tumor-infiltrating T cells across solid tumors and hematologic malignancies display evidence of exhaustion, including:
- Upregulation of PD-1 and other inhibitors of immune function10
- Decreased production of cytokines, the cell-signaling molecules that help guide the immune response10
- Impaired ability to kill tumor cells10
Modulating PD-1 reinvigorates exhausted T cells
Preclinical studies have shown that PD-1 blockade reinvigorates exhausted T cells and restores their cytotoxic immune function.3 Blocking the PD-1 pathway is an important area of continued research seeking to understand its impact on reversing T-cell exhaustion.
PD-L2 binding to PD-1 contributes to immune inhibition in cancer
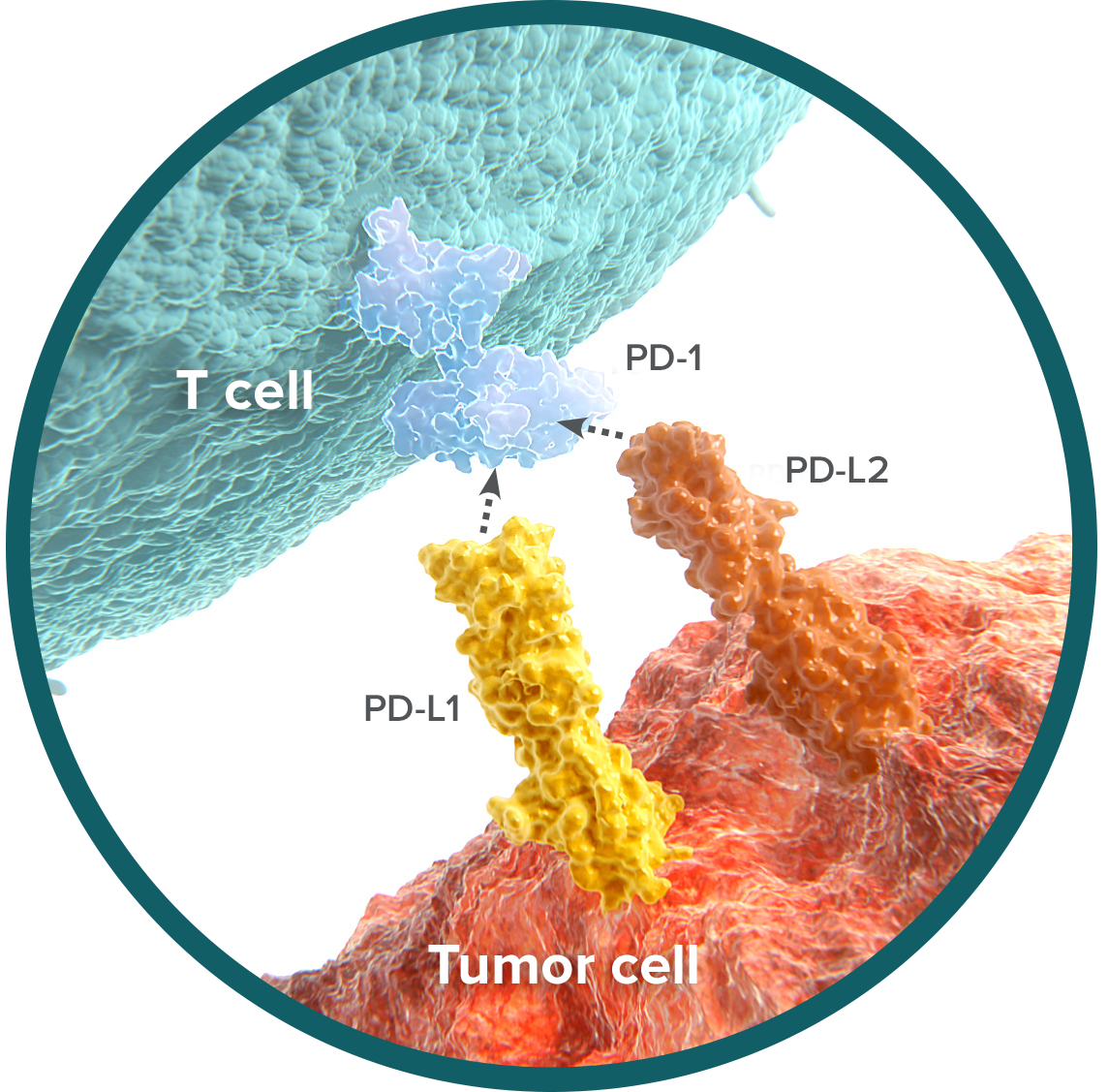
PD-L1 and PD-L2, the ligands for PD-1, are expressed on the surface of tumor cells.11 These ligands have overlapping functions in the ability to inhibit T-cell activity.1,2

Current data indicate the presence of PD-L2 in multiple solid tumors and hematologic malignancies, including renal cell cancer (RCC), melanoma, non-small cell lung cancer (NSCLC), esophageal cancer, pancreatic cancer, hepatocellular carcinoma, and lymphoma, suggesting a role for PD-L2 in tumor immune evasion.11-15
PD-1 has higher affinity for PD-L2 versus PD-L1
The role of PD-L1 in immune escape continues to be an area of active research. Close consideration of the interaction between PD-1 and its ligands has begun to elucidate the impact of PD-L2.1,2 When both ligands are present, PD-L2 is in close proximity to PD-L1, and the two ligands compete for binding to PD-1. In this situation, PD-1 has a preferential affinity for PD-L2 and likely forms a more stable complex with PD-1.11,16,17
Dual ligand inhibition achieves more complete PD-1 blockade—and may reinvigorate T cells more effectively
Preclinical studies suggest that complete inhibition of PD-1 signaling through both PD-L1 and PD-L2 may be more effective at reversing T-cell exhaustion than inhibiting PD-L1 alone.18
Research to further understand these pathways is ongoing.
References
1. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J Exp Med. 2000;192(7):1027-1034. 2. Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261-268. 3. Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682-687. 4. Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger LR. Activation-Induced Expression of Human Programmed Death-1 Gene in T-Lymphocytes. Exp Cell Res. 1997;232(1):25-28. 5. Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor Deficient Mice. Science. 2001;291(5502):319-322. 6. Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of Epitope Escape on PD-1 Expression and CD8 T-Cell Exhaustion during Chronic Infection. J Virol. 2009;83(9):4386-4394. 7. Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T Cell Responses by High Viral Loads. J Immunol. 2003;170(1):477-489. 8. Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence is acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362(6422):758-761. 9. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment. J Virol. 2003;77(8):4911-4927. 10. Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537-1544. 11. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti–PD-1 Therapy. Clin Cancer Res. 2014;20(19):5064-5074. 12. Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268-3377. 13. Hamanishi J, Mandal M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor infiltrating CD8 T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360-3365. 14. Nomi T, Sho M, Akahori T, et al. Clinical Significance and Therapeutic Potential of the Programmed Death-1Ligand/Programmed Death-1Pathway in Human Pancreatic Cancer. Clin Cancer Res. 2007;13(7):2151-2157. 15. Ohigashi Y, Sho M, Yamada Y, et al. Clinical Significance of Programmed Death-1 Ligand-1 and Programmed Death-1 Ligand-2 Expression in Human Esophageal Cancer. Clin Cancer Res. 2005;11:2947-2953. 16. Ghiotto M, Gauithier L, Serriari N, et al. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol. 2010;22(8):651-660. 17. Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307(3):672-677. 18. Hobo W, Maas F, Adisty N, et al. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen–specific CD8+ T cells. Blood. 2010;116(22):4501-4511.