Non-effector Cell Pathways
- PD-1 Pathway
- CTLA-4 Pathway
- Additional Effector T Cell
Pathways - SLAMF7 Pathway
- Additional NK Cell
Pathways - Non-effector Cell
Pathways
CSF1R: stimulates immunosuppression in the tumor microenvironment
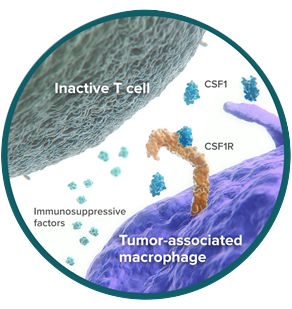
Colony-Stimulating Factor 1 Receptor (CSF1R) is a cell-surface receptor expressed by macrophages and other cells of the myeloid lineage.1

In the tumor microenvironment, some macrophages evolve from antitumor to protumor in their activity.2 These are called tumor-associated macrophages (TAMs). TAMs promote cancer survival and drive immunosuppression, supporting tumor growth.2
CSF1, the ligand for CSF1R, is a dominant regulator of macrophage differentiation and function.3 In cancer patients, high CSF1 concentrations are associated with poorer prognosis.3,4 Mouse models have shown that tumor cells use CSF1 to target CSF1R on macrophages, stimulating the development and survival of TAMs.5
In preclinical studies, blockade of CSF1R—depriving CSF1 of its target receptor—resulted in depletion of TAMs and improved T-cell responses.6,7
CD73: tipping the immune balance to a suppressive environment
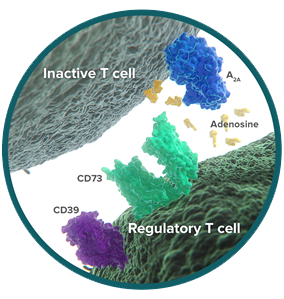
CD73 is a cell-surface enzyme on regulatory T cells (Tregs), where it is a critical checkpoint in the conversion of immune-activating ATP into immunosuppressive adenosine.8 Tregs act to limit the immune response, and the release of adenosine helps Tregs shut down immune activity.9,10

Cancer exploits the function of CD73 to reduce antitumor immunity. Like Tregs, tumor cells express CD73 and release adenosine into the tumor microenvironment.11-13 In cellular studies, adenosine powerfully inhibits the antitumor immune response, including proliferation and production of cytokines.8
Preclinical research has identified tumor-derived CD73 as a contributor to immune escape in cancer, and inhibition of CD73 activity can stimulate T-cell activity.14
IDO: depleting the fuel of immune cell survival
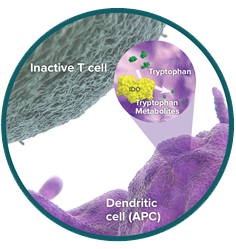
Indoleamine-2,3-dioxygenase-1 (IDO) is an intracellular enzyme that initiates the breakdown of tryptophan in the tumor microenvironment.15,16 Tryptophan is an amino acid essential for cell survival.17 Normally, tryptophan metabolism by IDO keeps T-cell immune responses under control.18

Tumor cells hijack this immunosuppressive process. They have evolved to upregulate IDO activity in order to suppress T-cell function and help themselves survive.19,20 Increased IDO has been linked to poorer outcomes in cancer.21,22
Tumor cells use IDO to induce immune tolerance by 2 distinct mechanisms:
- Directly, by starving tumor-reactive T cells of the tryptophan they need to survive and function18
- Indirectly, by triggering development of regulatory T cells (Tregs) that act to limit the immune response22,23
Based on preclinical studies, blockade of IDO can reduce the number of Tregs and restore cytotoxic T-cell function.22,24
Research to further understand these pathways is ongoing.
References
1. Stanley ER, Chitu V. CSF-1 Receptor Signaling in Myeloid Cells. Cold Spring Harb Perspect Biol. 2014;6:a021857. 2. Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41(1):49-61. 3. Richardson E, Uglehus RD, Johnsen SH, Busnd LT. Macrophage-Colony Stimulating Factor (CSF1) Predicts Breast Cancer Progression and Mortality. Anticancer Res. 2015;35(2):865-874. 4. Yang L, Wu Q, Xu L, et al. Increased expression of colony stimulating factor-1 is a predictor of poor prognosis in patients with clear-cell renal cell carcinoma BMC Cancer. 2015;15:67. doi:10.1186/s12885-015-1076-5. 5. Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res. 2014;74(18):5057-5069. 6. Ries CH, Cannarile MA, Hoves S, et al. Targeting Tumor-Associated Macrophages with Anti-CSF-1R Antibody Reveals a Strategy for Cancer Therapy. Cancer Cell. 2014;25:846-859. 7. Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting Tumor-Infiltrating Macrophages Decreases Tumor-Initiating Cells, Relieves Immunosuppression, and Improves Chemotherapeutic Responses. Cancer Res. 2013;73:1128-1141. 8. Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5’-adenosine monophosphate to adenosine. J Immunol. 2006;177(10):6780-6786. 9. Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257-1265. 10. Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5’-nucleotidase and nonspecific alkaline phosphatase. J Biol Chem. 2003;278(15):13468-13479. 11. Häusler SF, del Barrio IM, Strochschein J, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011;60(10):1405-1418. 12. Serra S, Horenstein AL, Vaisitti T, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118(23):6141-6152. 13. Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103(35):13132-13137. 14. Stagg J, Divisekera U, McLaughlin N, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA. 2010;107(4):1547-1552. 15. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20(10):469-473. 16. Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867-1870. 17. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762-774. 18. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363-1372. 19. Löb S, Königsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58(1):153-157. 20. Liu P, Xie BL, Cai SH, et al. Expression of indoleamine 2,3-dioxygenase in nasopharyngeal carcinoma impairs the cytolytic function of peripheral blood lymphocytes. BMC Cancer. 2009;9:416. doi:10.1186/1471-2407-9-416. 21. Jia Y, Wang H, Wang Y, et al. Low expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int J Cancer. 2015;137(5):1095-1106. 22. Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18(22):6110-6121. 23. Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752-6761. 24. Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269-1274.